Student Guide Sections
Introduction to Chemistry and Energy Efficiency
Insulation
Fertilizer and Crop Production
Lighting
Polymers
Fertilizer and Crop Production
According to the McKinsey report, agrochemicals are second only to insulation as a means of reducing GHG emissions. Chemical fertilizers and crop protection have reduced the amount of land converted to farming, which in turn has decreased the net amount of CO2 emitted by the chemistry industry. If these were not available, agricultural yield would decrease from 85 to 30 percent depending on crop type, soil, technology, and climate. Switching to organic farming for all crops would increase the CO2 emissions due to increased land required for an equivalent crop yield.
While both fertilizers and crop protection involve the chemistry industry, we will focus on fertilizers because the energy involved in production and use of pesticides for crop protection is only 6.6 percent of that associated with fertilizers.
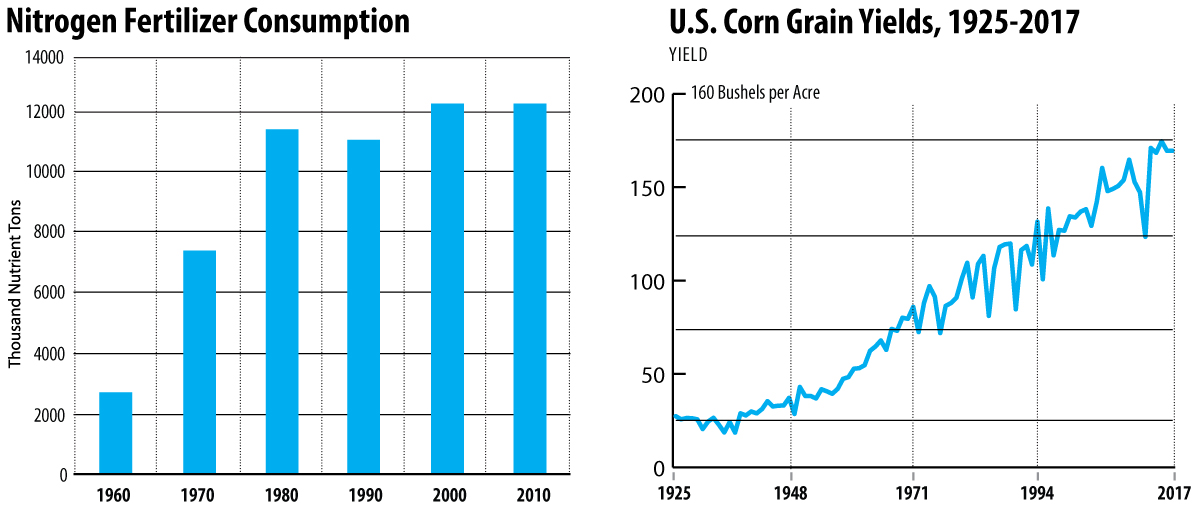
While phosphate and potassium components of fertilizers are important, we will focus on nitrogen-containing fertilizers. Half of the protein required to feed the world’s population is acquired from plant sources, and nitrogen content in fertilizers directly influences a plant’s ability to produce protein. Plants require nitrogen to produce this protein. Ammonia, NH3, is the only viable source of nitrogen for producing large amounts of protein. The nitrogen content of fertilizers improves both the quantity and quality of protein-containing crops. In addition to food production, nitrogen fertilizers are currently used to produce the plants for ethanol fuel. Ammonia ranks second behind sulfuric acid in volume of industrial chemical production with 85 percent going to fertilizers and the remainder used in the synthesis of pharmaceuticals and other products.
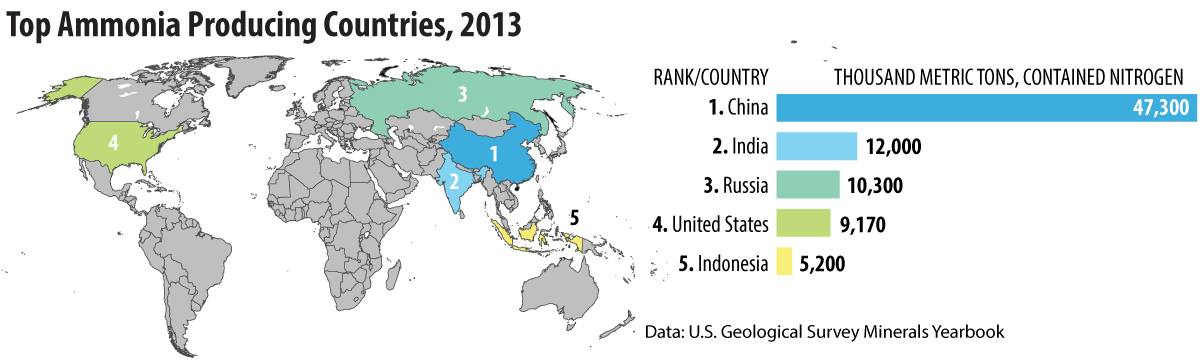
Ammonia is produced from nitrogen and hydrogen under high pressure in the presence of a catalyst. The reaction was discovered in Germany by chemist Fritz Haber shortly before World War I and was developed into an industrial process by Carl Bosch during the war to make explosives for Germany when other sources of materials were blockaded by the allies. Hydrogen is usually produced by reacting methane found in natural gas with steam in a two-step process.
CH4(g) + H2O(g) --> 3H2(g) + CO(g)
CO (g) + H2O (g) --> CO2 (g) + H2 (g)
Methane can also be produced by coal gasification.
Nitrogen is obtained by liquefying air and is then reacted with hydrogen to produce ammonia.
N2(g) + 3 H2(g) --> 2NH3(g)
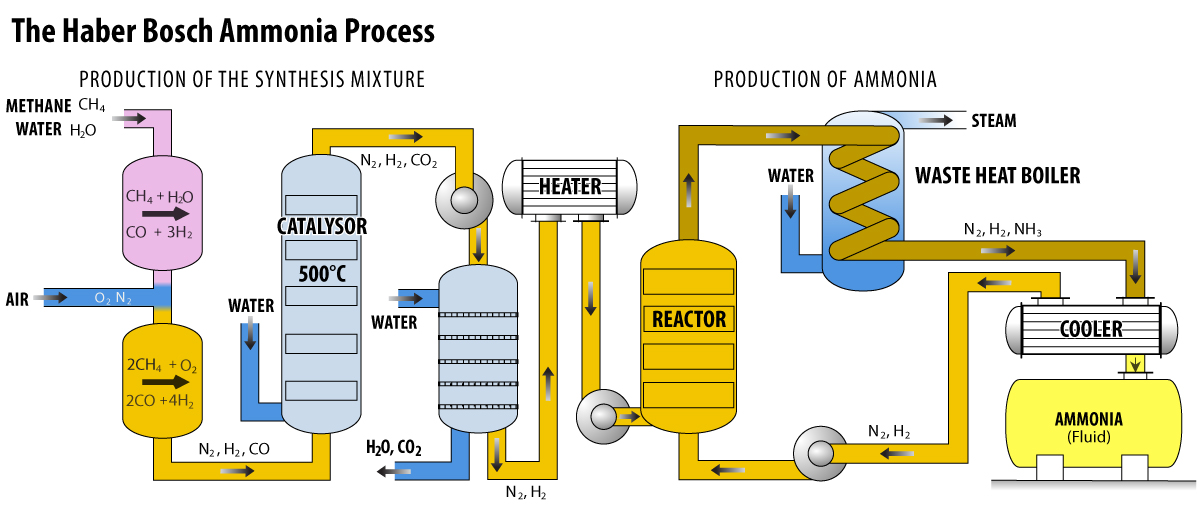
The synthesis of ammonia is one of the most energy intensive processes for producing industrial chemicals, requiring an estimated at 39.3 GigaJoules per tonne. The production of hydrogen from methane requires 16.7 GJ/tonne, and the remaining 22.6 GJ/tonne is accounted for in the production of carbon dioxide.
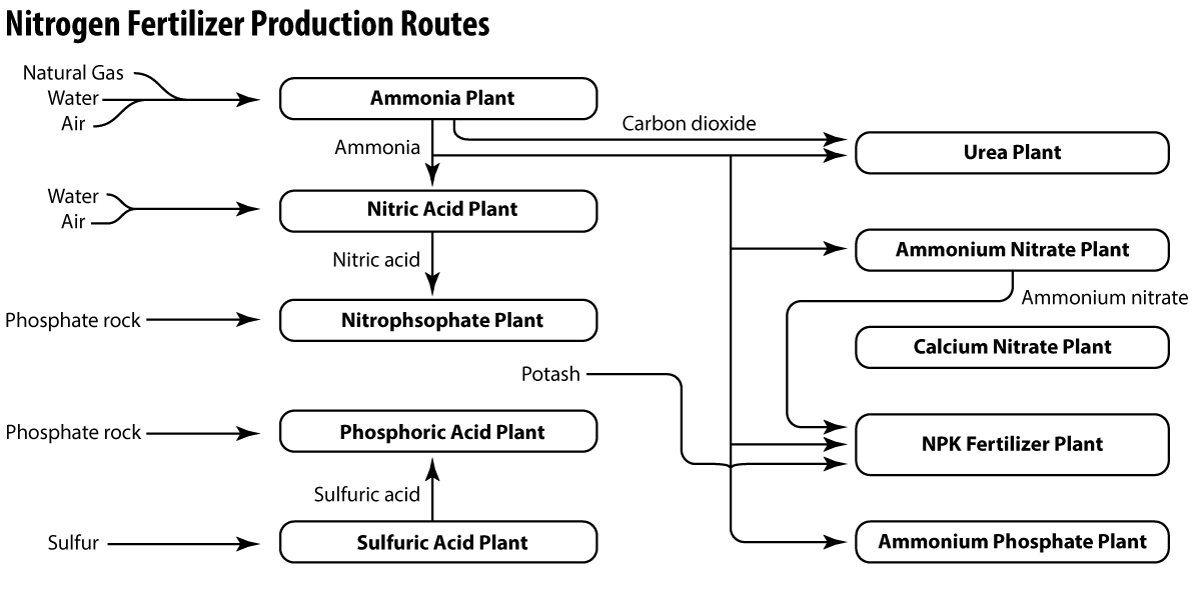
ImageWhile ammonia can be applied directly to the soil as a liquid or reacted with CO2 to produce urea ((NH2)2CO) fertilizer, a large percentage is converted to nitric acid (HNO3) by the Ostwald Processwhich uses platinum gauze as a catalyst. The nitric acid is then used to produce a variety of nitrate fertilizers including ammonium nitrate (NH4NO3), potassium nitrate (KNO3), and calcium nitrate (Ca(NO3)2). Ammonia is also used to produce ammonium phosphate (NH4PO4), and ammonium sulfate ((NH4)2SO4), which can also be used as fertilizers.
The McKinsey report focused on the five most important crops (corn, rice, soy, sugar cane, and wheat), which account for 56 percent of the world’s agrochemical use. Cultivation of these crops consumed 1,500 million acres of arable land. Changing to organic farming for these crops is estimated to require an additional 2,700 million acres (an increase of 183 percent) to achieve the same crop yield. If each acre used for farming increases CO2 emissions by 3.7 tons, the use of chemical fertilizers and crop protection potentially reduces CO2 emissions by 1,600 million tons as compared to strictly organic farming.
However, there is an environmental trade-off between using fertilizers to stabilize atmospheric carbon dioxide and producing oxygen depleted bodies of water. Rainwater dissolves unabsorbed chemical fertilizers and collects in drainage ditches and streams. The aqueous run-off from fields rich in nitrogen compounds used in fertilizer is the major cause of eutrophication which depletes the oxygen in rivers, lakes, and the oceans. The excess nutrients stimulate the growth of aqueous plants such as algae. When the plants die and decompose, high levels of organic matter and decomposing organisms deplete the surrounding water of oxygen causing the death of other organisms such as fish and crustaceans. Each spring the run-off from Midwestern farms flowing into the Mississippi river exacerbates dead zones in the Gulf of Mexico.