Student Guide Sections
Introduction to Chemistry and Energy Efficiency
Insulation
Fertilizer and Crop Production
Lighting
Polymers
Polymers
Without knowing anything at all about polymer chemistry, you can already learn something just from its name. The prefix “poly-“ means many. The Greek root “meros” means part. The word polymer literally means many parts.
Polymers are very large molecules made of repeating patterns of small molecules, called monomers. Many polymers are made by repeating the same small molecule over and over again. Others are made from two monomers linked in a pattern. While most polymers with which you are familiar are man-made, there are some biological polymers – proteins, nucleic acids, and complex carbohydrates – that are fundamental to the way your body functions. These can be made from one monomer all the way to twenty different small molecules coming together to form one large polymer. We are going to focus our attention on synthetic polymers from the chemistry industry.
Because they contain carbon, polymers are categorized as organic compounds. The most common element found in polymers, besides carbon, is hydrogen. Many polymers are manufactured from feedstock, or starting materials, obtained from petroleum. Petroleum is a mixture of hydrocarbon compounds pumped from underground and is the result of extreme time and pressure acting on ancient sea plants and animals. However, the compounds used to make polymers are not necessarily obtained directly from petroleum deposits. Petroleum must first be refined before it can be made into polymers. All polymers must then be manufactured through polymerization reactions. The two most common reaction types used to make polymers are addition reactions and condensation reactions.
In most addition polymerization reactions, hydrocarbons with double bonds, called alkenes, react with each other, breaking the double bond within the small molecules and forming a new covalent bond between the two monomers. Ethylene, C2H4, is commonly used and is combined to make polyethylene.
ImageVarying reaction conditions and the type of catalyst used will result in different structures of polyethylene polymers. One method uses high temperature and pressure with a peroxide catalyst and results in low-density polyethylene, LDPE. Another method, called Ziegler-Natta polymerization, takes place at lower temperature and pressure and produces high-density polyethylene, or HDPE.
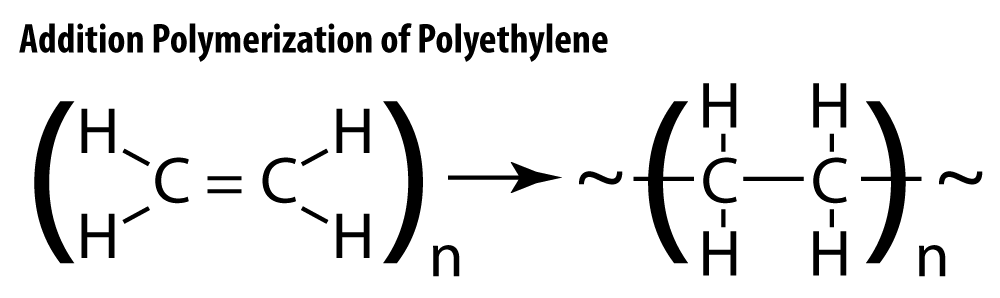
LDPE and HDPE are both made from the same monomer, ethylene, yet have very different physical properties. The differences in their properties is a direct consequence of the degree of crystallization found among the polymer molecules. Molecules that are highly branched, such as LDPE, cannot “line up” with each other. The result is an Imageamorphous solid – one that does not have a repeating, predictable structure. As the molecule becomes less and less branched, and more and more long-chain-like, the intermolecular attraction between them becomes stronger, and the structure takes on more of a crystalline appearance. Crystalline solids have a regular, repeating arrangement. LDPE has many branches, and is less chain-like in structure. It is softer, more flexible, and more easily deformed than HDPE. HDPE molecules are much longer, less branched, packed more closely together, and have stronger intermolecular attraction than LDPE. The variations in their properties lend them to different, albeit equally useful, applications. LDPE is used to make soft items like grocery store bags and clear plastic food wrap, and HDPE is used in more rigid applications like detergent bottles and toys.
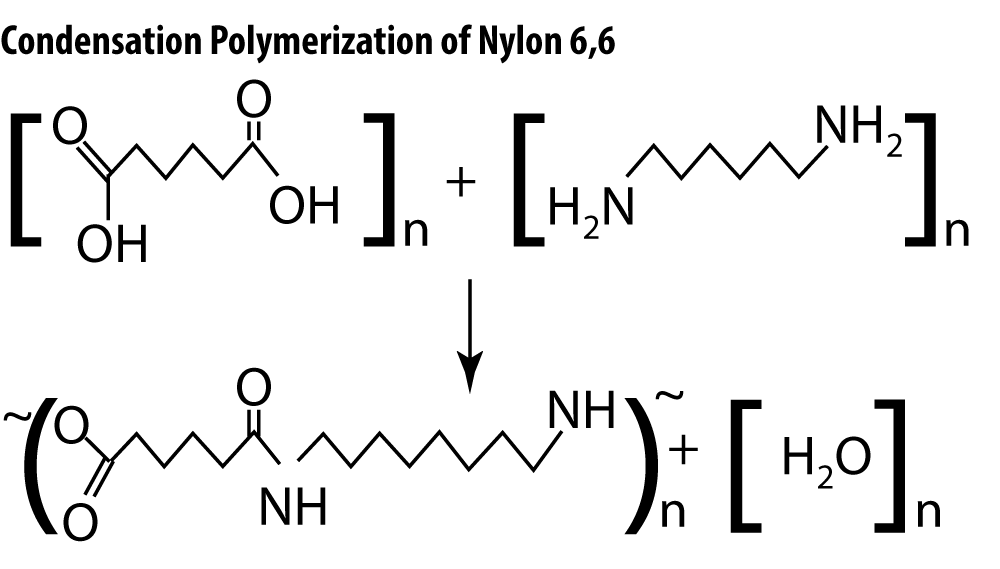
Condensation reactions occur between molecules that contain oxygen along with hydrogen and carbon. Two hydrogen-containing functional groups must be present on each molecule for the reaction to proceed.
Note that in the polymerization of Nylon 6,6, a hydroxyl group (-OH group) is removed from one molecule and a hydrogen atom is removed from the other. The two molecules may be joined to form a dimer, and those dimers combined to form a polymer, or the polymer may be built monomer by monomer. Either way, a water molecule is formed as a byproduct.
Besides nylon, some common polymers formed via condensation include: polyester, a textile fiber; polycarbonate, a lightweight material used in eyeglasses, molded sign faces, and NASCAR seats; Teflon, a non-stick coating on cookware; Kevlar, the material used in bullet-proof vests; and, polyurethane, used in many foam applications.
Plastics
Plastics are polymers made from petrochemicals. When plastics are used for packaging rather than steel, aluminum, glass, paper, or wood, the net emissions saved are 222 MtCO2e. Polymers can also be used to replace glass in agricultural green houses, in window frames instead of wood and aluminum frames, and in carpeting. All of these uses of plastics can reduce greenhouse gas emissions.
Automotive Industry
The automotive industry uses polymers, such as carbon fiber reinforced polymers, in the design and manufacturing of vehicles. Polymers have a wide range of uses in automobile manufacturing. They are used in the chassis, under-the-hood, in the body, and in the interior. Using polymer based materials reduces the weight of the vehicle, which reduces fuel consumption, which reduces GHG emissions. The McKinsey Report estimates that the use of plastics in the automotive industry saves about 120 MtCO2e from entering the atmosphere.
Plastics in Piping
Most people are familiar with PVC and HDPE piping. The plastics used in these pipes are polymers created by the chemistry industry. When compared to different metal pipe options, the lifetime of plastic pipes are similar. Savings in GHG emissions come from lower raw material use, and differences in production, and disposal footprints. Overall, plastics in piping has a net emissions savings of 65.4 MtCO2e.
Electronics
The chemistry industry is working on new uses for polymers as well. In development are conductive polymers for printable electronics. Polymer Electrolyte Membrane, PEM, (or Proton Exchange Membrane) fuel cells are already in use in hydrogen fuel cell vehicles. The industry is also working on materials for advanced fuel cells including a polymer electrolyte fuel cell (PEFC).