Teacher Guide Sections
Introduction to Chemistry and Energy Efficiency
Insulation
Fertilizer and Crop Production
Lighting
Polymers
Suggested Reading: Introduction to Chemistry and Energy Efficiency
Activity One: Chemicals in Your Life
Objective
Students will begin to think about the role the chemical industry plays in their lives.Time
One class periodMaterials
-Chemistry and Energy Efficiency QuizProcedure
- Before students do any reading, have students take the Chemistry and Energy Efficiency Quiz.
- Show students the picture below. In three to five minutes have students list as many items, events, activities that can be associated with the chemical industry.
- Discuss with the class what they listed. Does everyone agree with what was chosen? Save the list to use at the end of the module.

Activity Two: Chemical Hazard Placards
Objective
Students will learn how chemicals are labeled to identify hazards.Time
One class periodMaterials
-Chemical Hazard Placard worksheetsProcedure
- Review chemicals students are familiar with. Discuss what Students know about chemical safety.
- Pass out Chemical Hazard Placard worksheets. Students should follow directions to create their own placards.
Activity Three: Modeling Greenhouse Gases
Objective
Students will understand why carbon dioxide, methane, and water are three of the major greenhouse gases.Time
One class periodMaterials
-Molecular Models kit with spring attachments to act as bondsProcedure
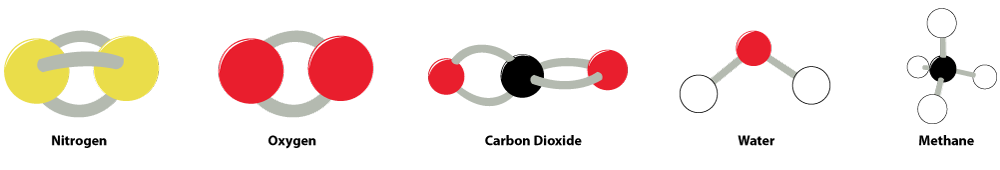
- Explain to your students: Carbon dioxide (CO2), methane (CH4) and water (H2O) are three of the major greenhouse gases. When the radiant energy from the sun travels through the Earth’s atmosphere and strikes the surface of the Earth, much of that energy is radiated back as thermal energy and infrared energy that will leave the atmosphere, but much of it is absorbed by the greenhouse gases. The Earth’s atmosphere is composed of around 78 percent nitrogen gas (N2), which has a triple bond between the nitrogen atoms, and 21 percent oxygen gas (O2), which has a double bond between the oxygen atoms. In order for molecules to store thermal energy, the energy is stored within the movement of those molecules. Nitrogen gas and oxygen gas do not have a great deal of flexibility in the vibrations, rotation, expansion, and contraction of the bonds within the molecule.
- Demonstrate to students how there is a great deal of flexibility in the bonds of water, methane, and carbon dioxide, while there is very little in the flexibility of the other gases in the atmosphere. While holding the central atom in the structures of water (O), carbon dioxide (C), and methane (C), apply a slight force to the atoms attached to the central atom and show how the bonds are able to move freely indicating the ability to store more energy than the bonds within nitrogen gas or oxygen gas.
Activity Four: Greenhouse in a Beaker
Objective
Students will understand that carbon dioxide speeds up the transfer of thermal energy.Time
One class periodMaterials
For Each Group:- 2 600 mL Beakers
- 1 250 mL Flask
- 1 Rubber stopper with hole
- 1 Vinyl tubing, 3/16” diameter, 60 cm long
- 1 Clip light with 1 75 watt bulb
- 1 Ruler
- 2 digital thermometers
- 1 Small piece masking tape
- 4 Alka-Seltzer tablets
- Safety glasses
- Copies of Greenhouse in a Beaker for each student
- Water (room temperature)
Procedure
- Introduce the investigation to students by asking, “What affect does adding carbon dioxide to the air have on the air’s temperature?”
- Explain that students will be creating two models of our atmosphere (air inside of the beakers), and the lamp represents the sun. One beaker will contain a “normal” atmosphere. Carbon dioxide (CO2) will be added to the second beaker, creating a CO2 rich atmosphere. The CO2 will be produced through a chemical reaction that occurs when Alka-Seltzer is added to water. The active ingredients in Alka-Seltzer are aspirin, citric acid, and sodium bicarbonate (NaHCO3). When the tablet is placed in water, an acid-base reaction involving sodium bicarbonate and the citric acid takes place yielding the following products which include water and carbon dioxide. 3NaHCO3 + C6H8O7 --> C6H5Na3O7 + 3CO2 + 3H2O
- Divide students into small groups. Pass out the Greenhouse in a Beaker worksheets.
- Circulate around the room assisting groups as needed.